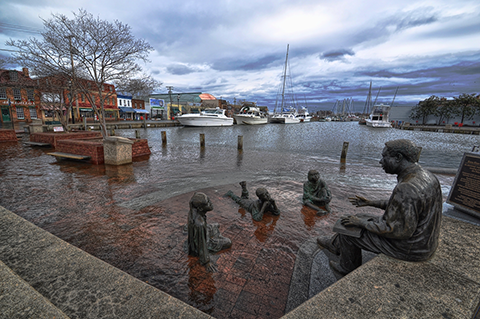
On Tuesday, November 19, NOAA sea level rise expert William Sweet answered questions in a Climate.gov tweet chat about sea level rise and U.S. high-tide flooding.

NOAA is an agency that enriches life through science. From the surface of the sun to the bottom of the ocean, NOAA advances scientific understanding of Earth's environment, climate, and weather.
Differences in exposure to sunlight, cloud cover, atmospheric circulation patterns, and other factors influence whether and how much a location is warming or cooling.
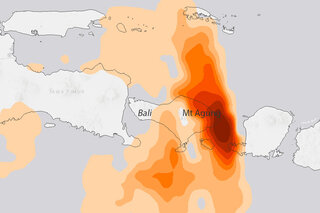
Mount Agung erupts in Indonesia: Is it a climate event?
December 7, 2017
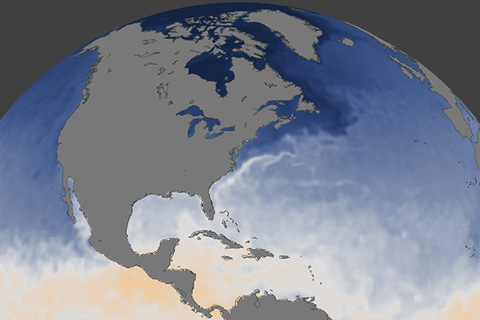
NOAA's Climate Prediction Center releases its 2017 Atlantic hurricane outlook, saying odds favor an above-normal hurricane season.
Mild days—perfect for an outdoor picnic or a nice hike—are expected to drop about 14 percent by the end of the century, according to new research.

Trees within a city can help reduce urban heat, control stormwater, and provide habitat to local wildlife. As climate conditions change, a Chicago group is working to enhance the reilience of the city's urban forest.

Northwest Passage clear of ice again in 2016
September 16, 2016

Climate connections to Fort McMurray fire
May 12, 2016
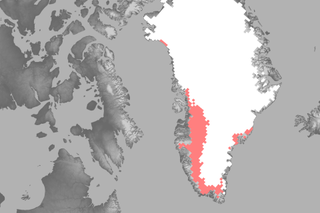
Greenland melt season off to very early start
April 28, 2016